Antioxidant 3.0: How the Amino Acid Combo GlyNAC Made 80-Year-Olds Function Like 20-Year-Olds
The Best Antioxidant is Produced in the Body, Not the Factory
Over the years, we have been bombarded with marketing messages about antioxidants in our food and supplements, promising to protect us from various ailments and aging. However, our readers know better. Welcome to the era of "Antioxidant 3.0," where the most potent antioxidants are generated from within.
In a previous article, we explored the delicate balance between antioxidants and oxidative stress, and showed that exogenous direct antioxidants may not be the panacea we once believed. Instead, harnessing our body's innate ability to produce antioxidants and shuttling them into cells "just in time" could allow for more precise control over our antioxidant levels.
Enter GlyNAC, a specific combination of two amino acids that has recently gained attention for its ability to reverse five of the nine hallmarks of aging. Comprising glycine and the antioxidant N-acetylcysteine, GlyNAC empowers our bodies to produce their own antioxidants, promoting a more natural, balanced response to oxidative stress.
Dr. Premranjan Kumar, a researcher at the forefront of GlyNAC studies, has discovered that this unique amino acid combination not only boosts our internal antioxidant production but also rejuvenates the physical and cognitive functions of 80-year-olds to levels similar to those of 20-year-olds. By promoting our body's natural antioxidant production, GlyNAC may offer an effective solution to the challenges posed by conventional antioxidant supplementation.
In this article, we will delve into the world of GlyNAC, examining its impact on aging and its potential to address the unique challenges faced by specific populations, such as older adults and vegans. We will also explore the current state of research on this remarkable compound and consider its implications for the future of antioxidant therapy.
II. Glutathione: The Master Antioxidant Tripeptide and its Decline with Age
A. Definition and composition
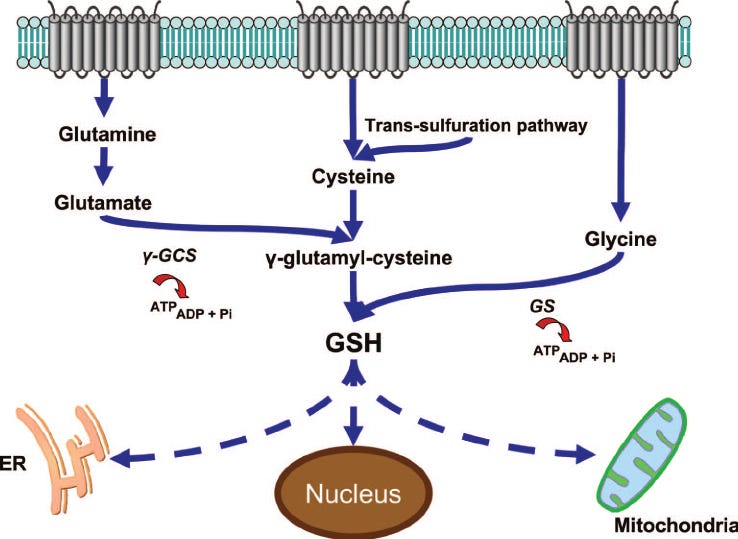
To understand GlyNAC, we must first understand glutathione. Glutathione, or GSH, is often referred to as the "master antioxidant" due to its unique properties and extensive reach within the body. It is involved in detoxification processes, immune system support, and the neutralization of free radicals. Unlike other antioxidants that may target specific areas or functions, glutathione works systemically, protecting cells, tissues, and organs throughout the body. Additionally, it has the ability to regenerate other antioxidants, such as vitamins C and E, making it even more valuable in combating oxidative stress ( Wu et al., 2004).
Figure 1 - The central role of glutathione in the pathophysiology of human disease
Source: The central role of glutathione in the pathophysiology of human disease - Scientific Figure on ResearchGate.
GlyNAC is a combination of glycine and N-acetyl cysteine (NAC), two of the three precursors required to create the tripeptide glutathione, (see figure 1). The third precursor is glutamate, which is abundant in most people, particularly in the central nervous system, and is involved in various aspects of normal brain function and cellular metabolism (Meldrum, 2000). Therefore, glutamate's abundance will not be a limiting factor and will not be discussed further in this article.
Glycine, an endogenous amino acid (meaning that the body can create its own supply from other amino acids), is a fundamental building block for collagen, which makes up around one-third of our skin, hair, bones, and joints. According to a study published in the American Journal of Clinical Nutrition, the average adult produces around 3g of glycine per day whereas the estimated daily requirement for optimal collagen synthesis is around 13g (Meléndez-Hevia 2009). Yet most people do not consume enough glycine-rich foods like animal skin and tendon to fill this gap. As we age, our collagen production significantly declines, resulting in a 75% loss by the time we're 80 compared to our 20s (Varani et al., 2006).
Glycine also plays a significant role in childbirth and pregnancy. It is involved in the synthesis of essential proteins, nucleic acids, and other molecules necessary for the formation of the placenta, fetal membranes and tissues. Adequate glycine levels are essential for a healthy pregnancy, as it helps support proper placental function and may reduce the risk of complications such as fetal loss and low birth weight. In this context, it is incredibly important for pregnant individuals to maintain sufficient glycine levels through diet or supplementation (Tan, Chengquan, et al. 2022).
The other important raw material for creating GSH is cysteine, which is where NAC comes in. NAC is a precursor of cysteine and is involved in numerous cellular processes. It protects cells from drugs and chemicals and has been linked to a decreased risk of cancer, diabetes, respiratory issues, and infertility. Additionally, cysteine plays a crucial role in the formation of keratin, an essential structural protein in hair, skin, and nails.
B. GlyNAC's role in boosting glutathione production
Given glutathione's status as the "master antioxidant”, many people make the mistake of supplementing glutathione directly, which may not be as effective as supplementing its precursors: glycine and NAC (GlyNAC). The real advantage of GlyNAC lies in its ability to support cells in producing their own glutathione. As different cells in different organs require different amounts of glutathione at different times, supplementing GlyNAC allows cells to maintain their own glutathione levels, which is essential for balancing between fighting oxidative stress and avoiding reductive stress.
To be more precise, GlyNAC's mechanism of action involves supporting the synthesis of glutathione (GSH) within the cell, which neutralizes reactive oxygen species (ROS) and prevents oxidative stress. GSH is a tripeptide because its synthesis requires three amino acids: glutamate, glycine, and cysteine. Interestingly, supplementation with glycine or NAC alone does not increase lifespan, while the combination of the two boosts it. Direct GSH supplementation also decreases lifespan, potentially due to inducing reductive stress.
One notable comparison to make is with other well known antioxidant supplements, such as omega-3 fatty acids. While omega-3 has been hailed for its numerous health benefits, a recent mRNA research has shown that the popular antioxidant supplement may accumulate in the skin, triggering an immune response that causes hair loss due to oxidative stress (Hao 2022). An antioxidant that leads to more oxidation? Yes, a paradox that may be familiar to our early subscribers!
In other words, by providing the necessary precursors for "just-in-time" glutathione synthesis, GlyNAC supports the body's natural ability to maintain optimal levels of this critical antioxidant, promoting overall health and well-being.
C. Who Benefits Most from GlyNAC Supplementation?
So who might benefit the most from GlyNAC? The answer is: older people, people with chronic diseases, and vegetarians or vegans.
As we age, glutathione levels naturally decline, making older adults more vulnerable to the ramifications of this deficiency. The difference is massive. Young 20-year-olds have 4x higher glutathione levels than 80-year-old people (Kumar 2021).
Second, people with chronic diseases often have elevated inflammation levels, which can continuously deplete their glutathione stores. As a result, they may require more glutathione to maintain optimal health and mitigate the negative effects of their conditions.
Third, vegetarians and vegans, who often adopt low-protein diets and are lacking amino acids like glycine and cysteine, face an elevated risk of glutathione deficiency (Hoffman 2004). Research has demonstrated that vegans possess markedly lower glutathione levels compared to their omnivore (and lacto-ovo vegetarian) counterparts (Elorinne 2016).
The lower levels of glycine and cysteine in vegan diets could potentially contribute to certain health challenges. Consequently, GlyNAC supplementation proves advantageous for vegans and those following low-protein diets, addressing both glutathione deficiency and amino acid shortages. While low-protein diets have been associated with increased longevity, they may also result in diminished energy levels, glutathione deficiency, and potentially hair loss due to protein deficiency (Rushton 2002) — making GlyNAC a vital component for these groups.
III. GlyNAC's Remarkable Ability to Target the Hallmarks of Aging
Aging is a complex process driven by multiple factors that contribute to the gradual deterioration of our bodies. Researchers have pinpointed nine hallmarks of aging that play a significant role in this decline, including genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication.
GlyNAC has shown promise in targeting and reversing five of these nine hallmarks. In this section, we will explore the extent of the impact on these hallmarks and discuss its implications for longevity and health.
A. The Astonishing Results of GlyNAC in Mice Trials
In the study, scientists gave a group of mice GlyNAC, starting when they were middle-aged, and continued giving it to them for the rest of their lives. This resulted in the mice living significantly longer—about 24% more than they normally would. So why did this happen? Let's break it down.
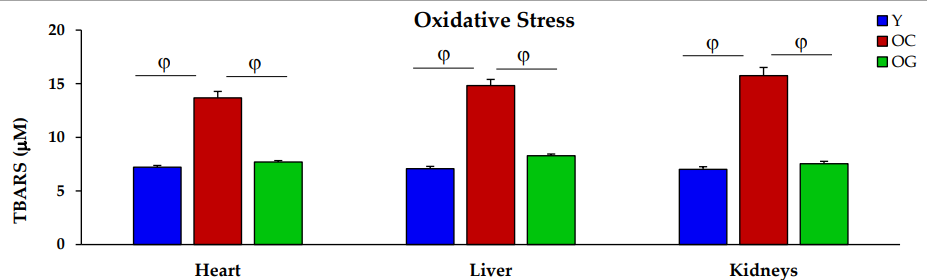
Oxidative stress: Oxidative stress occurs when there is an imbalance between free radicals and antioxidants in the body. Older mice receiving GlyNAC experienced a reduction in oxidative stress, returning to levels seen in younger mice.
Figure 2: The impact of GlyNAC supplementation on oxidative stress, as indicated by TBARS levels in young mice (Y) vs. old mice on a control diet (OC) and old mice on GlyNAC supplemented diet (OG) at the beginning (65 weeks) and end (90 weeks) of supplementation.
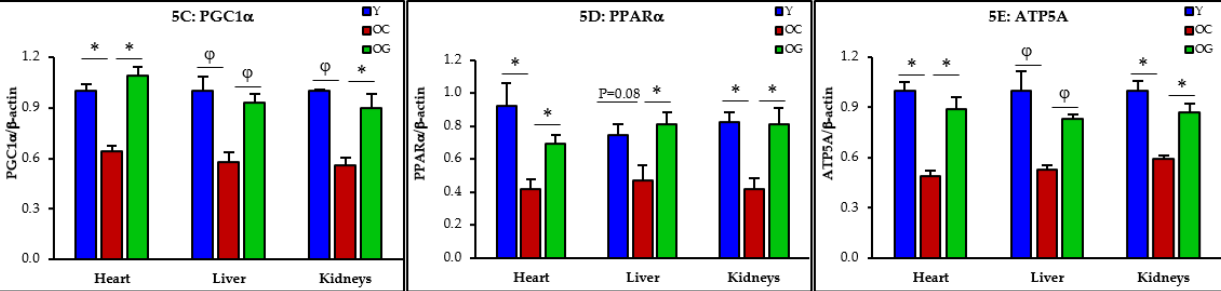
Mitochondrial function and mitophagy: Mitochondria are the energy-producing structures within cells, while mitophagy is the process of removing damaged mitochondria. GlyNAC improved mitochondrial function and mitophagy in older mice, restoring them to the levels observed in their younger counterparts.
Figure 3: The impact of GlyNAC supplementation on mitochondrial function and mitophagy, as indicated by PPARG coactivator 1 alpha (PGC1α), Peroxisome proliferator-activated receptor alpha (PPARα), mitochondrial ATP synthase (ATP5A), and PTEN-induced kinase 1 (PINK1) in young mice (Y) vs. old mice on a control diet (OC) and old mice on a GlyNAC supplemented diet (OG) at the beginning (65 weeks) and end (90 weeks) of supplementation.
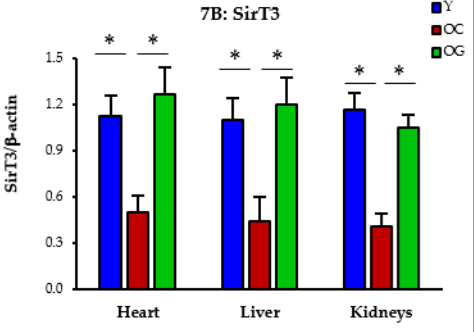
Nutrient sensing: Nutrient sensing is a cell's ability to detect and respond to changes in nutrient availability. GlyNAC treatment restored proper nutrient sensing in older mice, bringing it back to youthful levels.
Figure 4: The impact of GlyNAC supplementation on nutrient sensing, as indicated by sirtuin 3 (SirT3) levels in young mice (Y) vs. old mice on a control diet (OC) and old mice on GlyNAC supplemented diet (OG) at the beginning (65 weeks) and end (90 weeks) of supplementation.
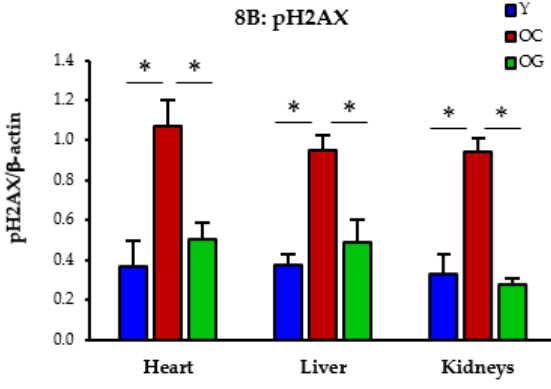
DNA damage: DNA damage refers to changes in the genetic material of cells, which can lead to mutations and other problems. Older mice given GlyNAC exhibited a decrease in DNA damage, with their genomic stability returning to levels found in younger mice.
Figure 5: The impact of GlyNAC supplementation on oxidative stress, as indicated by phosphorylated H2AX (pH2AX) levels in young mice (Y) vs. old mice on a control diet (OC) and old mice on GlyNAC supplemented diet (OG) at the beginning (65 weeks) and end (90 weeks) of supplementation.
So, why did all these positive changes happen? It appears that GlyNAC helps fix a combination of age-related issues, such as low levels of the crucial antioxidant GSH, oxidative stress, problems with the energy-producing parts of cells called mitochondria, issues with the process of removing damaged mitochondria (mitophagy), irregularities in how cells sense nutrients, and damage to the genetic material (DNA) inside cells.
Not only did GlyNAC improve the health of the mice's vital organs, but it also showed promise in human trials, which we’ll cover below. But first, how does GlyNAC compare to existing longevity interventions? And what makes it different?
B. GlyNAC vs. Other Anti-aging Interventions
GlyNAC is a unique compound, as it can simultaneously increase lifespan and vitality. This is an unusual feat since most remedies face a direct trade-off between these two objectives.
For instance, calorie restriction combined with fasting remarkably boosts mice lifespan by 35% (Acosta-Rodríguez et al., 2022), while rapamycin achieves a 15% increase (Strong et al., 2020). However, these interventions have drawbacks: calorie-restricted mice show reduced body size and slower growth, while rapamycin-treated mice experience impaired glucose metabolism and insulin resistance (Lamming 2012). Both interventions slow cellular growth and metabolism by inhibiting mTOR, a protein regulating cell growth. In some cases, such as with rapamycin, this can also suppress the immune system, potentially impacting overall health and vitality (Janes & Fruman, 2009).
For readers familiar with longevity research, metformin needs no introduction. This type 2 diabetes drug has been shown to extend mice lifespan by 6% (Martin-Montalvo et al., 2013). However, it does so by starving the body of glucose and ramping up sugar metabolism via AMPK activation, which can compromise athletic performance as glucose is the key fuel source (Konopka et al., 2019).
Similarly, 17-alpha-estradiol, a form of estrogen, has been found to increase male mice lifespan by 12% by suppressing testosterone production (Wink et al., 2022). While this may work for mice, it's worth noting that reduced testosterone can negatively affect men's physical, sexual, and emotional health.
Another emerging molecule in the longevity field is calcium alpha-glutarate (Ca-AKG), which we discussed in an earlier substack post. Ca-AKG has been shown to increase female mice lifespan by 17% (10% in males) when started later in life, equivalent to 60 human years (Su et al., 2019).
Like Ca-AKG, GlyNAC does not compromise the immune system, athletic performance, or hormonal balance. Instead, it boosts mouse lifespan by a remarkable 24% (even when started in midlife, equivalent to 50 human years). This is why GlyNAC is garnering attention in the scientific community – as of 2023, no other intervention has demonstrated such a notable ability to reverse aging while simultaneously increasing vitality.
C. Why Now?
A question that may come to mind is why now? Why not wait until there's more evidence on GlyNAC? As with any research, quality of evidence is more important than quantity. By examining not only the endpoints but also what drove the results, we gain a better understanding of GlyNAC's potential impact on the aging process.
One of the critical questions to consider is: "did glutathione levels increase through GlyNAC supplementation?". This inquiry is essential because the primary mechanism of action for GlyNAC is improving age-associated glutathione deficiency. If we can establish that glutathione levels increased, we have a solid foundation to build upon.
D. From Mice to Humans: Clinical Trial at the Baylor College of Medicine
The human trial conducted at the Baylor College of Medicine, a reputable institution located in Houston, Texas, sought to answer the question – whether GlyNAC increases glutathione levels – and many others. The 36-week open-label trial involved eight 80-year-olds and eight 20-year-olds (and replicated in a 2023 randomized controlled trial involving thirty-six people). The hypothesis was to determine whether supplementing 100 mg/kg/day of GlyNAC for 24 weeks in older adults would improve age-associated glutathione deficiency, as well as physical health as measured by over 30 biomarkers and real-life performance differences.
The results of the trial were surprising: all categories of measurements seem to suggest that GlyNAC may have rejuvenated the bodies of 80-year-olds back to levels resembling 20-year-olds after 24 weeks of GlyNAC supplementation.
Besides increasing glutathione levels, the trial also found that oxidative stress decreased, mitochondrial fuel oxidation improved, inflammation improved, and endothelial function improved. Additionally, the trial showed reductions in DNA damage, insulin resistance, and improvements in cognitive function and physical performance.
Moreover, the study is strongly supported by the 2022 mice trial at Baylor College of Medicine, which found a whopping 24% increase in lifespan for mice supplemented with GlyNAC. This sister paper also reported a reversal of five hallmarks of aging, similar to the two human trials.
Let’s zoom in.
IV. GlyNAC's Clinical Trial Results Surprised the (Longevity) World
So did glutathione levels increase with GlyNAC supplementation? And how does it impact other major biomarkers of health and aging? It’s time to put GlyNAC to the test.
A. Putting GlyNAC to the Test: Impact on 7 Biomarkers
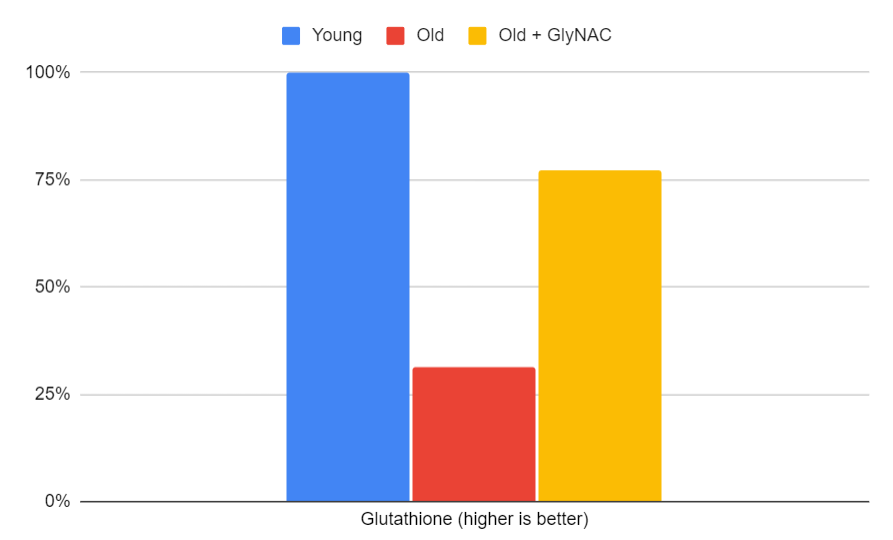
Glutathione Levels
Glutathione, a potent antioxidant, is essential for cellular protection and detoxification. The trial revealed that 80-year-olds began with 76% lower levels of glutathione than 20-year-olds. However, after 24 weeks of GlyNAC supplementation, glutathione levels increased 2.6-fold and were restored to those of their younger counterparts, as measured by reduced GSH and total GSH. The accompanying bar graph will illustrate these significant changes.
Figure 6: The effects of GlyNAC supplementation on glutathione, as indicated by the average of total and reduced GSH levels in young vs. old adults at the beginning (0 week) and end (24 weeks) of supplementation.
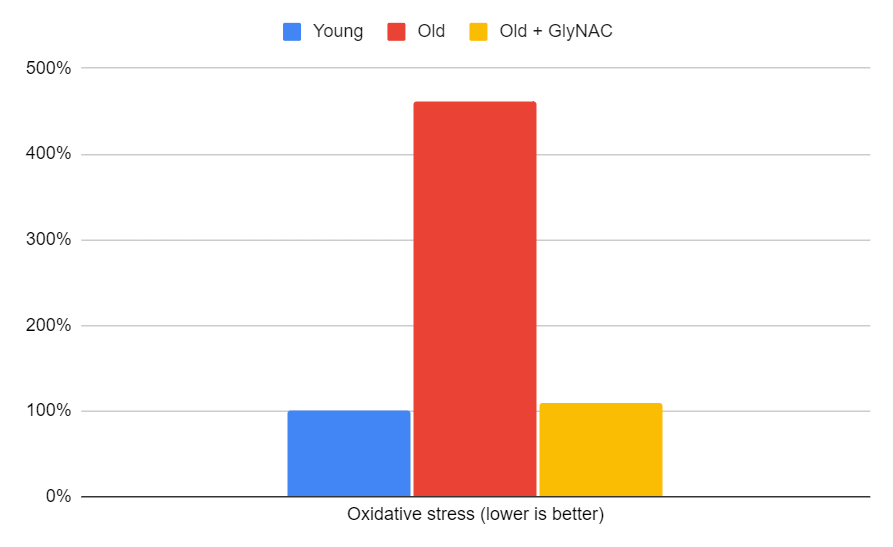
Oxidative Stress
A critical factor to consider is the reduction of oxidative stress, which is closely tied to glutathione levels. The trial found that 80-year-olds initially had 5-10 times higher oxidative stress levels, as measured by blood levels of TBARS and F2-isoprostane. With GlyNAC supplementation, the average of these two markers was fully restored to youthful levels. The process of cellular protection requires the detoxification of hydrogen peroxide (H2O2) to water, which relies on GSH. By correcting the glutathione deficiency, oxidative stress levels were effectively lowered.
Figure 7: The impact of GlyNAC supplementation on oxidative stress, as indicated by the average of TBARS and F2-isoprostane levels in young vs. old adults at the beginning (0 weeks) and end (24 weeks) of supplementation.
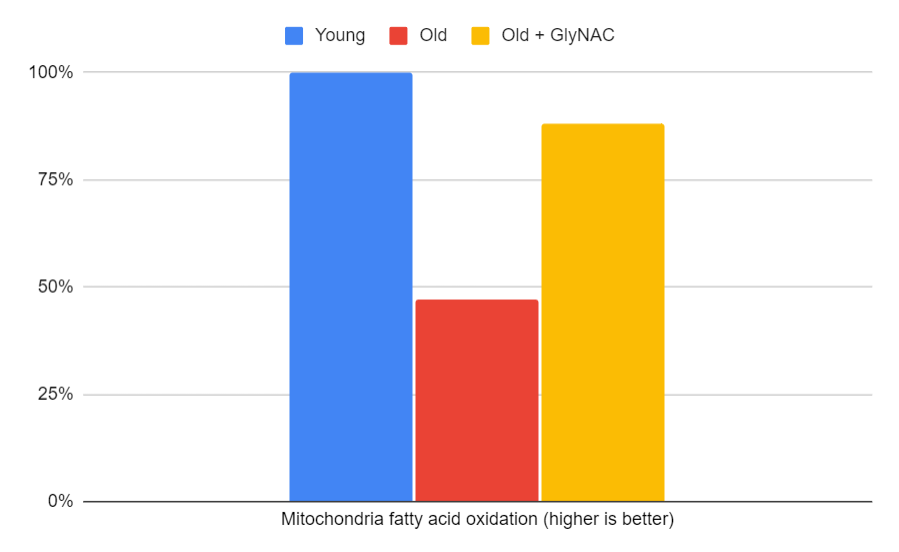
Mitochondrial Fuel Oxidation
Mitochondrial fuel oxidation, responsible for breaking down fat for fuel, is another crucial aspect of aging. The trial demonstrated that 20-year-olds were significantly more efficient at fat metabolism, with 80-year-olds operating at a much lower efficiency, approximately half that of their younger counterparts. GlyNAC supplementation remarkably restored fat metabolism in older adults, bringing it close to the efficiency levels seen in 20-year-olds. This finding is especially significant because it suggests that GlyNAC could offer a novel nutritional approach to improve and correct mitochondrial dysfunction in older adults and possibly other human conditions.
Figure 8: The impact of GlyNAC supplementation on fasting mitochondria fatty acid oxidation levels in young vs. old adults at the beginning (0 weeks) and end (24 weeks) of supplementation.
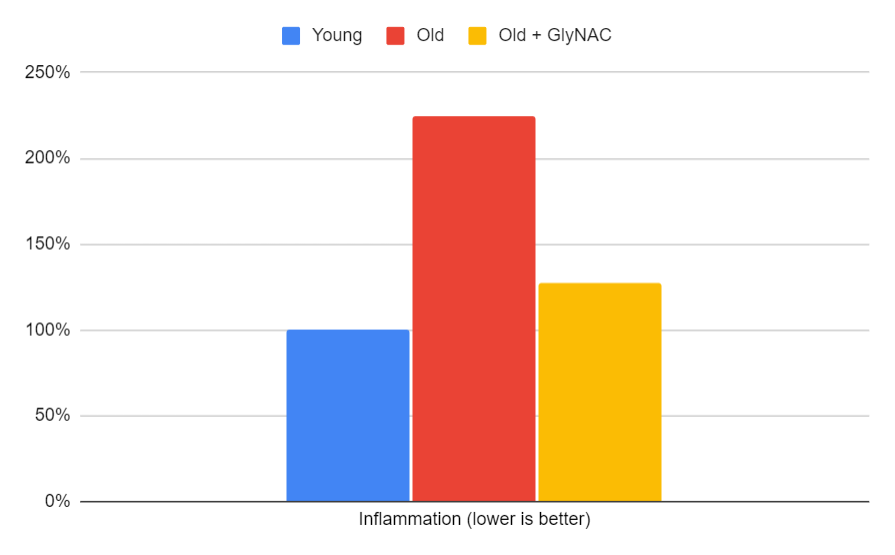
Inflammation
When it comes to inflammation, the trial measured three different markers, all of which were about twice as high in older adults. After 24 weeks of GlyNAC supplementation, inflammation levels in the older participants returned to similar levels as the 20-year-olds. The average across these markers is displayed in the accompanying bar graph.
Figure 9: The impact of GlyNAC supplementation on inflammation, as indicated by the average of interleukin-6 (IL6), tumor necrosis factor alpha (TNFa), and c-reactive protein (CRP) levels in young vs. old adults at the beginning (0 weeks) and end (24 weeks) of supplementation.
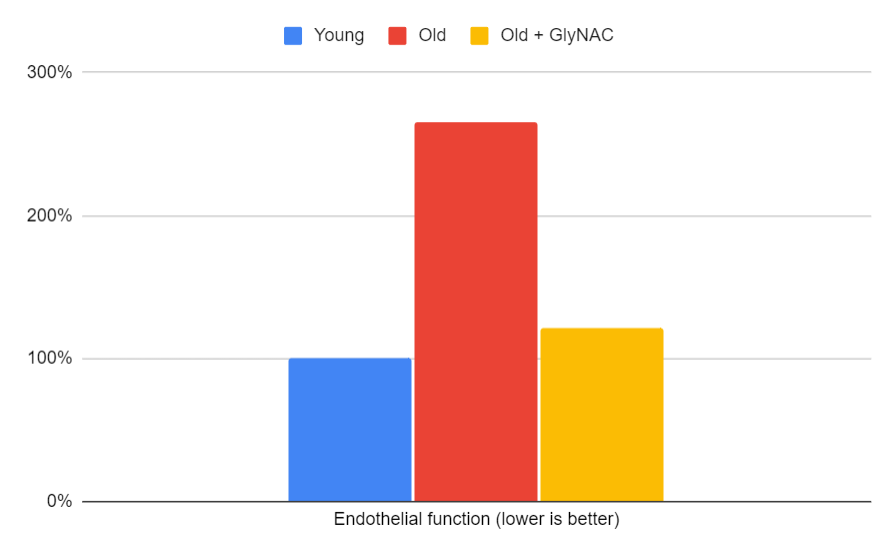
Endothelial Function
Endothelial function, which measures the integrity of blood vessels, also improved markedly back to youthful levels. The trial measured three biomarkers related to endothelial function, and the average of these improvements will be illustrated in the graph.
Figure 10: The impact of GlyNAC supplementation on endothelial function, as indicated by the average of sICAM1, sVCAM1, and E-selectin levels in young vs. old adults at the beginning (0 weeks) and end (24 weeks) of supplementation.
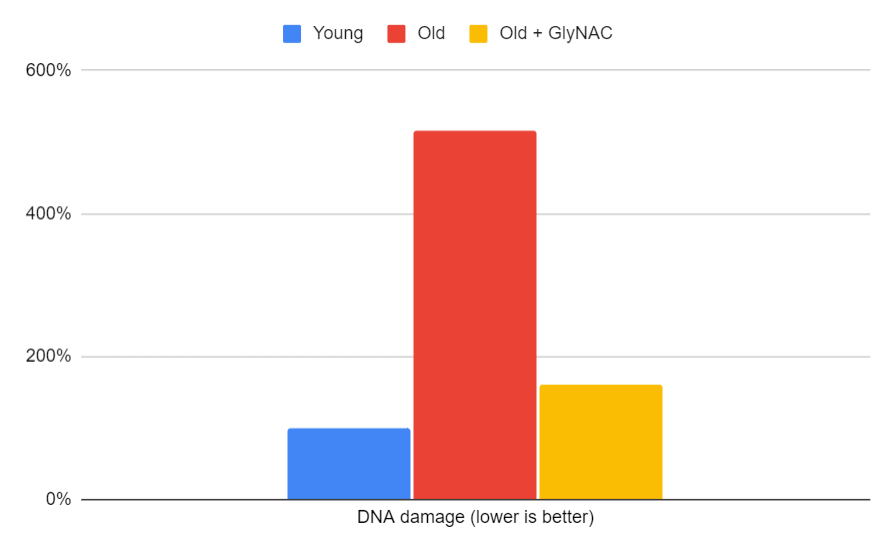
DNA Damage
DNA damage is another important factor in the aging process. The trial confirmed that 80-year-olds have higher levels of DNA damage through a validated biomarker of DNA damage, 8-OHdG (8-hydroxydeoxyguanosine). Increased DNA damage and oxidative stress have been linked to an elevated risk of cancer. GlyNAC supplementation lowered oxidative stress and 8-OHdG to near youthful levels. While further studies are needed to establish a direct effect on cancer, several studies have found that glycine supplementation prevents the development of hepatic tumors and inhibits the growth of melanoma and mammary adenocarcinoma.
Figure 11: The impact of GlyNAC supplementation on DNA damage, as indicated by 8-hydroxy deoxyguanosine levels in young vs. old adults at the beginning (0 weeks) and end (24 weeks) of supplementation.
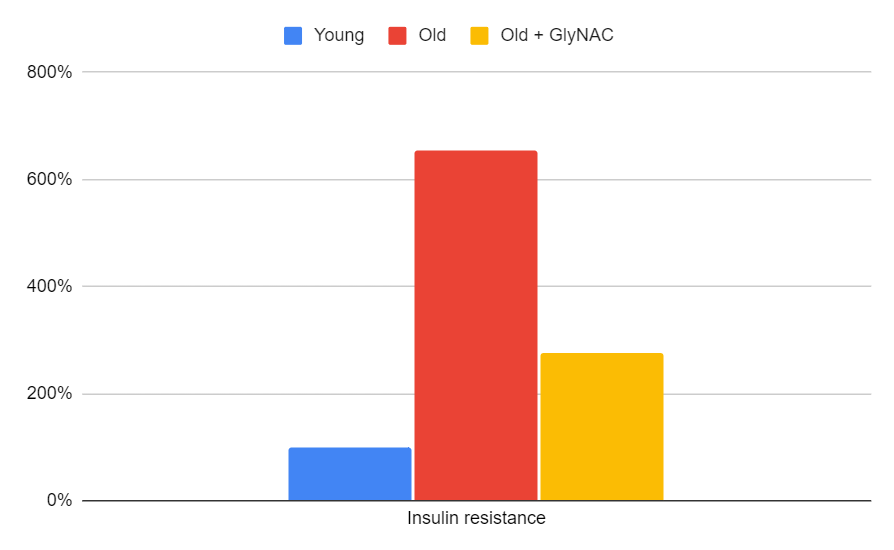
Insulin Resistance
Insulin resistance is another key measure to consider. The trial found that GlyNAC corrected elevated insulin resistance levels in older adults. This improvement has well-established links to a reduced risk of metabolic syndrome, atherosclerosis, and cardiovascular disease. Furthermore, a 2022 mice study by Kumar showed that GlyNAC supplementation corrected cardiac inflammation and diastolic dysfunction, providing additional support for its potential benefits.
Figure 12: The impact of GlyNAC supplementation on insulin resistance, as indicated by Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) levels in young vs. old adults at the beginning (0 weeks) and end (24 weeks) of supplementation.
B. Did Improved Biomarkers Actually Make A Difference In The Real World?
Alright, we've seen that the cells are happy, but what about the humans themselves? In other words, did all of these improved biomarkers actually translate into real-world cognitive and physical enhancements?
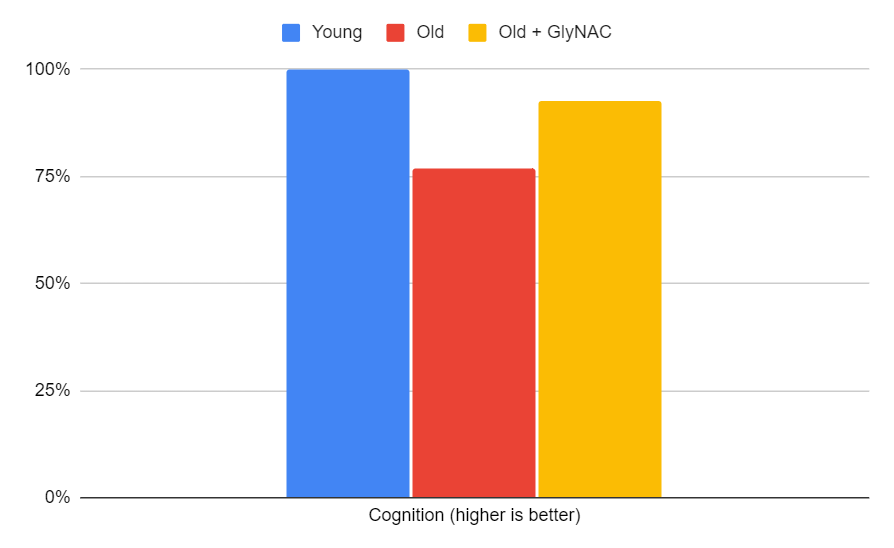
Cognitive Improvements
Cognitive function improvement is one of the most exciting results of the trial. Five cognitive tests were performed, and BDNF (brain-derived neurotrophic factor) levels were measured. Initially, 80-year-olds had 36% lower BDNF levels. with GlyNAC supplementation, not only increased BDNF levels 3-fold, but the levels were so thoroughly restored in older participants that they became indistinguishable from those of younger participants. This increase in BDNF led to better test scores, bringing the performance nearly on par with the younger 20-year-old participants. The trial offers several explanations for this cognitive improvement:
The "glucose-steal phenomenon": With GlyNAC supplementation, non-brain tissues revert to using fat as fuel, resulting in a fall in whole-body mitochondrial glucose oxidation. This leaves more glucose available for the brain, thus improving cognition.
Improved insulin resistance: Elevated insulin resistance could limit glucose entry into the brain. Improved insulin resistance has been linked to enhanced cognition.
Brain inflammation: Cognitive impairment has been associated with brain inflammation and endothelial dysfunction. GlyNAC supplementation may help alleviate this inflammation.
BDNF: The significant increase in BDNF after GlyNAC supplementation could have improved memory, synaptic plasticity, and maintenance of neural networks.
Figure 13: The impact of GlyNAC supplementation on cognition, as indicated by the average of Montreal cog, Trails (A & B average), Verbal fluency, Digital symbol substitution (% completion and % accuracy average), and BDNF levels in young vs. old adults at the beginning (0 weeks) and end (24 weeks) of supplementation.
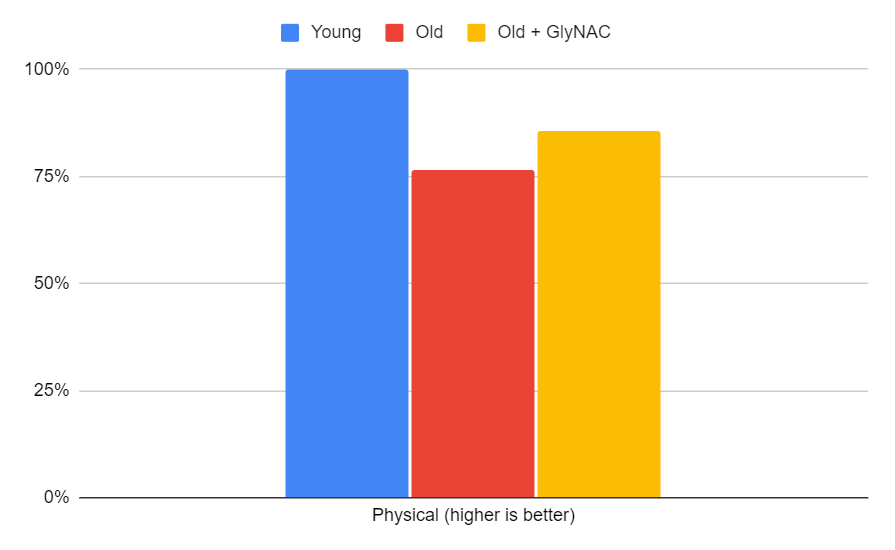
Physical Performance
Lastly, let's examine the physical performance of the participants. Both young and old were tested on physicals, including gait speed, 6-minute fast walk, and grip strength. As expected, the older adults initially performed 23% worse than their younger counterparts. However, after GlyNAC supplementation, their physical ability improved to within 14% of the younger participants.
The trial suggests that GlyNAC improves overall physical function by potentially enhancing mitochondrial function, which is linked to strength and gait speed. However, stopping GlyNAC led to a reversal of accrued benefits in most parameters, suggesting that continued supplementation may be required to maintain these benefits.
Figure 14: The impact of GlyNAC supplementation on physical function, as indicated by the average of Gait speed, 6-min rapid walk, and Grip strength (dominant and non-dominant average) levels in young vs. old adults at the beginning (0 weeks) and end (24 weeks) of supplementation.
GlyNAC Is Well Tolerated with No Side Effects
It is worth noting that no negative side effects were reported from GlyNAC supplementation, and no participants withdrew from the trial. The researchers also saw no elevation in plasma creatinine, alanine transaminase, or aspartate transaminase, which are relevant safety measures.
This human trial offers promising evidence that GlyNAC supplementation can correct multiple hallmarks of aging by restoring glutathione deficiency, improving oxidative stress and mitochondrial dysfunction, and positively impacting glycine and cysteine levels. While further trials are needed to bolster these findings, GlyNAC may pave the way for innovative nutritional interventions to help older adults age more gracefully.
V. Practical Considerations for GlyNAC Supplementation: Dosage and Interactions
When considering GlyNAC supplementation, it is essential to take into account the appropriate dosages and forms of the compound. GlyNAC is typically taken as a combination of its two constituent amino acids, glycine and N-acetylcysteine. The optimal dosage of GlyNAC may vary depending on factors such as age, health status, and specific health goals. As a starting point, you might consider the dosages used in clinical trials, such as the one conducted by Kumar et al. (2021), where participants received 100mg/kg/day of glycine and 100mg/kg/day of N-acetylcysteine daily.
It is important to be mindful of potential interactions between GlyNAC and other medications or supplements. N-acetylcysteine, for example, may interact with nitroglycerin, a medication used to treat chest pain (angina), potentially leading to low blood pressure and dizziness. Additionally, GlyNAC supplementation may affect the absorption or efficacy of certain medications, such as tetracycline antibiotics or antacids containing aluminum. To minimize the risk of interactions, you should consult with your healthcare provider before beginning GlyNAC supplementation, particularly if you are taking any medications or other supplements.
Conclusion: GlyNAC - A Promising Path to Healthy Aging and Longevity
In conclusion, GlyNAC's potential to address the antioxidant paradox and counteract the hallmarks of aging is a groundbreaking scientific discovery that has captivated both researchers and health enthusiasts. This compound, a mix of glycine and N-acetylcysteine, circumvents the trade-offs commonly associated with anti-aging interventions, offering a cutting-edge nutritional approach without compromising immune function or athletic performance.
The human study successfully rejuvenated 80-year-olds to resemble their 20-year-old selves, based on measured biomarkers and real world physical and cognitive performance, indicating GlyNAC's potential to reverse age-related declines. This achievement is further bolstered by a mice study that restore five hallmarks of aging back to youthful levels, suggesting the human trial's findings are not mere anomalies. Impressively, GlyNAC extended mice lifespan by a record-breaking 24% for a non-prescription, nutraceutical intervention.
The results are undeniably promising, but please consult your healthcare professional for potential interactions with medications or other supplements. Aging is a complex, individualized process, and while GlyNAC holds considerable promise, it is only one piece of the puzzle. Until we solve the entire puzzle, GlyNAC emerges as a beacon of hope, offering the potential for a healthier, more vibrant life.
–
If you found value in today's discussion, please take a moment to like, subscribe, comment, and share. Your engagement helps me to continue creating content that matters to you. As always, our topics are chosen by you, our readers. So if you have an idea or topic you'd like to see covered, let me know in the comments below. Until next time, stay healthy, be kind, and know that your support is deeply appreciated.



References
Kumar, Premranjan, et al. "Glycine and N‐acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: results of a pilot clinical trial." Clinical and translational medicine 11.3 (2021): e372.
Kumar, Premranjan, Ob W. Osahon, and Rajagopal V. Sekhar. "GlyNAC (Glycine and N-Acetylcysteine) Supplementation in Mice Increases Length of Life by Correcting Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Abnormalities in Mitophagy and Nutrient Sensing, and Genomic Damage." Nutrients 14.5 (2022): 1114.
Kumar, Premranjan, et al. "Supplementing glycine and N-acetylcysteine (GlyNAC) in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, physical function, and aging hallmarks: a randomized clinical trial." The Journals of Gerontology: Series A 78.1 (2023): 75-89.
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217.
Elorinne, Anna-Liisa, et al. "Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians." PloS one 11.2 (2016): e0148235.
Hoffman, Jay R., and Michael J. Falvo. "Protein–which is best?." Journal of sports science & medicine 3.3 (2004): 118.
Rushton, D. Hugh. "Nutritional factors and hair loss." Clinical and experimental dermatology 27.5 (2002): 396-404.
Hao, Jiaqing, et al. "Consumption of fish oil high-fat diet induces murine hair loss via epidermal fatty acid binding protein in skin macrophages." Cell Reports 41.11 (2022): 111804.
Meldrum, B. S. (2000). Glutamate as a neurotransmitter in the brain: review of physiology and pathology. The Journal of nutrition, 130(4S Suppl), 1007S-15S.
Salminen, A., Kaarniranta, K., Kauppinen, A., & Ojala, J. (2021). Age-related changes in AMPK activation: role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Research Reviews, 66, 101249.
Sekhar, R. V., Patel, S. G., Guthikonda, A. P., Reid, M., Balasubramanyam, A., Taffet, G. E., & Jahoor, F. (2011). Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. The American Journal of Clinical Nutrition, 94(3), 847-853.
Sekhar, R. V., McKay, S. V., Patel, S. G., Guthikonda, A. P., Reddy, V. T., Balasubramanyam, A., & Jahoor, F. (2020). Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care, 34(1), 162-167.
Sadowska-Bartosz, I., & Bartosz, G. (2014). Effect of antioxidants supplementation on aging and longevity. BioMed research international, 2014.
Wu, G., Fang, Y. Z., Yang, S., Lupton, J. R., & Turner, N. D. (2004). Glutathione metabolism and its implications for health. The Journal of Nutrition, 134(3), 489-492.
Varani, James, et al. "Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation." The American journal of pathology 168.6 (2006): 1861-1868.
Glycine
Razak, Meerza Abdul, et al. "Multifarious beneficial effect of nonessential amino acid, glycine: a review." Oxidative medicine and cellular longevity 2017 (2017).
Meléndez-Hevia, Enrique, et al. "A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis." Journal of biosciences 34 (2009): 853-872.
Tan, Chengquan, et al. "A review of the amino acid metabolism in placental function response to fetal loss and low birth weight in pigs." Journal of Animal Science and Biotechnology 13.1 (2022): 28.
Wang, W., Wu, Z., Dai, Z., Yang, Y., Wang, J., & Wu, G. (2013). Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids, 45(3), 463-477.
Cysteine
Rushworth, G. F., & Megson, I. L. (2014). Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacology & Therapeutics, 141(2), 150-159.
Samuni, Yuval, et al. "The chemistry and biological activities of N-acetylcysteine." Biochimica et Biophysica Acta (BBA)-General Subjects 1830.8 (2013): 4117-4129.
Zafarullah, M., Li, W. Q., Sylvester, J., & Ahmad, M. (2003). Molecular mechanisms of N-acetylcysteine actions. Cellular and Molecular Life Sciences CMLS, 60(1), 6-20.
Lifespan references
Acosta-Rodríguez, Victoria, et al. "Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice." Science 376.6598 (2022): 1192-1202.
Martin-Montalvo, Alejandro, et al. "Metformin improves healthspan and lifespan in mice." Nature communications 4.1 (2013): 2192.
Konopka, Adam R., et al. "Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults." Aging cell 18.1 (2019): e12880.
Strong, Randy, et al. "Rapamycin‐mediated mouse lifespan extension: late‐life dosage regimes with sex‐specific effects." Aging Cell 19.11 (2020): e13269.
Janes, Matthew R., and David A. Fruman. "Immune regulation by rapamycin: moving beyond T cells." Science signaling 2.67 (2009): pe25-pe25.
Su, Yuan, et al. "Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK." Aging (Albany NY) 11.12 (2019): 4183.
Wink, Lily, Richard A. Miller, and Gonzalo G. Garcia. "Rapamycin, Acarbose and 17α-estradiol share common mechanisms regulating the MAPK pathways involved in intracellular signaling and inflammation." Immunity & Ageing 19.1 (2022): 1-20.
thanks, lots in the works so stay tuned